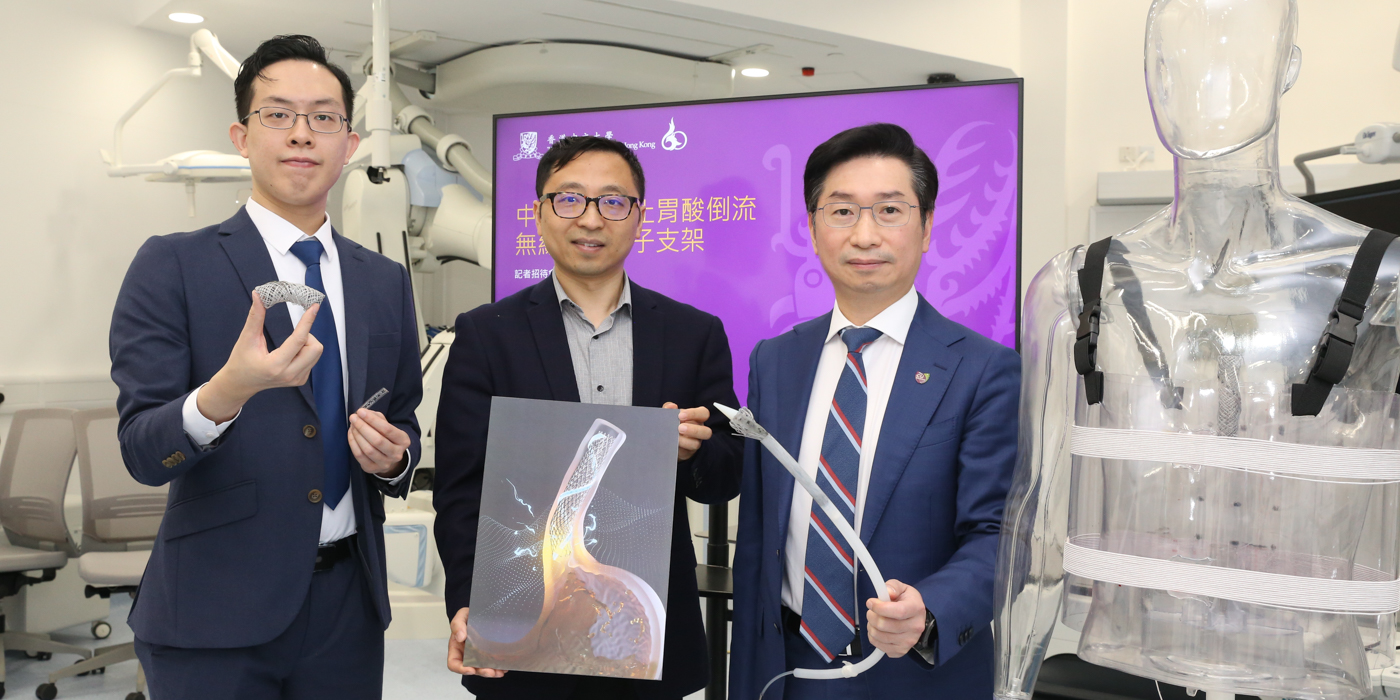
(From left) Professor Tony Chan Kai-fung of CU Medicine, Professor Zhang Li of the Department of Mechanical and Automation Engineering, and Professor Philip Chiu Wai-yan of CU Medicine
A novel non-invasive therapy for gastric acid reflux treatment
A collaborative research team led by professors from the Faculty of Engineering and the Faculty of Medicine (CU Medicine) has developed wirelessly powered electronic stents for a new electrical stimulation therapy to prevent and potentially cure gastric acid reflux. This technological breakthrough has been published in the renowned international research journal Science Advances, and will be highlighted by Nature Reviews Bioengineering in April 2023 issue.
Gastric acid reflux and the limitations of current therapeutic methods
Gastroesophageal reflux disease (GERD) is persistent and hard to cure. About 8% of patients need life-long medication, which impairs both their physical and mental health. Apart from medication, current surgical interventions include Nissen fundoplication and magnetic augmentation, which passively enhance the closure of the lower esophageal sphincter (LES) and require laparoscopic surgery. Alternatively, electrical stimulation of the LES provides a promising strategy to restore the normal functions of the LES without affecting swallowing.
However, it needs invasive surgery that is associated with a risk of infection. The heavy surgical burden and potential risks prevent the widespread implementation of implantable electrical stimulation systems. With the increasing prevalence of GERD patients worldwide, there is a strong demand for a less invasive electrical stimulation system to minimise invasive surgery-induced risks and increase the acceptance of electrical stimulation among GERD patients.
Eliminating the need for invasive surgery to implant the device and replace batteries
The wirelessly powered electronic stent (E-Stent) designed by the research team has overcome the bottleneck of powering bioelectronic implants and provides a transoral delivery and retrieval strategy for in situ diagnosis and treatment in the gastrointestinal tract. It also has the potential for other non-invasive biomedical applications in organs with a natural orifice.

The E-Stent consists of a super elastic clinical esophageal stent as the mechanical skeleton, a liquid metal antenna and an intrinsically stretchable pulse generator. The liquid metal has a low melting temperature of 15.4℃ and high electrical conductivity. Due to its liquid nature at body temperature, the liquid metal antenna is compliant with deformations in the esophagus. In cooperation with a wearable power transfer system designed by the research team, the elastic antenna can harvest sufficient energy for electrical stimulation therapy through deep tissue, even under compression of the esophagus. To further push technology transfer, the research team also exploited a laser engrafting machine for the batch fabrication of stretchable circuits, which improves design flexibility and speeds up production. Its intrinsic stretchability and excellent mechanical properties mean the circuit can provide stable biphasic current stimulation under various extreme deformations.
Professor Philip Chiu Wai-yan, Director of the Chow Yuk Ho Technology Centre for Innovative Medicine, commented, “The natural orifice procedure via the mouth offers less invasive access to the GI tract, which provides great benefits for diagnosis and surgical interventions. This work offers a bioelectronic platform to regulate GERD by electrical stimulation. We proved our concept in vivo in pig models: that continuous electrical stimulation increased the pressure on the lower esophageal sphincter, suggesting it has the potential to prevent gastric acid in a less invasive way.”
Professor Zhang Li, Professor in the Department of Mechanical and Automation Engineering, added, “In this collaborative work with CU Medicine, we have proposed the E-Stent platform and a transoral delivery strategy to address several key challenges in mucosa-interfacing bioelectronics: for instance, how to wirelessly power bioelectronics inside the body, especially for applications like electrical stimulation that consume a lot of power, and how to design microneedle electrodes to provide efficient, safe electrical stimulation across the mucosa, which is the natural barrier protecting the GI tract.”
Professor Tony Chan Kai-fung, Research Assistant Professor of the Chow Yuk Ho Technology Centre for Innovative Medicine, remarked, “Digital medicine can have a transformative impact on our daily life and demonstrates the clinical potential to integrate other functions. For example, we can integrate pressure sensors and other bio-sensors with the E-Stent for physiological assessment. The data we collect can be used for real-time optimisation of the electrical stimulation, realising personalised treatment.”
The research team is now working closely to integrate new functions with the E-Stent for other applications in the GI tract and to conduct further preclinical studies and clinical evaluations. The team envisions that the development of the E-Stent will provide a promising non-invasive platform offering high efficiency and safety for various physiological assessments and personalised treatments, as well as diverse functions with high clinical value.